Batch Release Testing
The EU batch release and import of biological IMPs (cell therapy and “others”) the EU batch release and import of veterinary sterile and nonsterile medicinal products;.

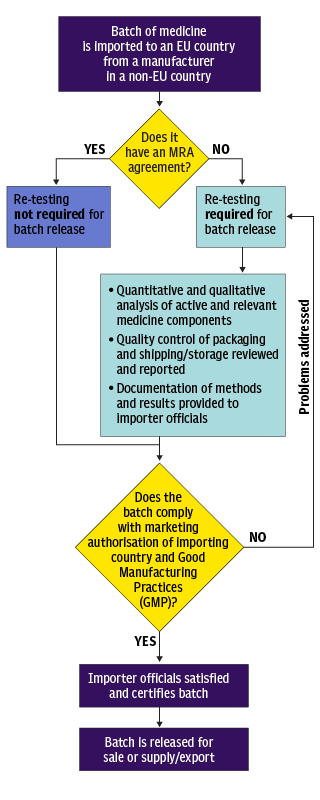
Batch release testing. Batch release testing and EU retests Conducting batch release analysis on behalf of a client is synonymous with partnership, communication, trust and control – but also with the many opportunities that become available for the design of the project QPcertified batch release analysis. The process of batch release comprises of i The checking of the manufacture and testing of the batch in accordance with defined release procedures ii The certification of the finished product batch performed by a QP signifying that the batch is in compliance with GMP and the requirements of its MA This. BATCH RELEASE MRA NO MRA RETESTING REQUIRED FOR BATCH RELEASE BATCH IS RELEASED FOR SALE OR SUPPLY/EXPORT PROBLEMS ADDRESSED LIFE SCIENCE I TECHNICAL BULLETIN 3 that have an MRA with the EU6 If a medicinal product batch is imported in separate parts, either conditions of.
Pharmaceutical lot and batch release testing is an ongoing critical aspect of drug development, from earlystage through commercial batch release As a key component of the quality control process, release testing is necessary to ensure biopharmaceutical drug substances, drug products, raw materials and inprocess materials meet established specifications prior to final product release. Batch testing is the process of confirming every batch of medicine has the correct composition through laboratory tests QP certification and release is the confirmation that the batch meets the. Batch Release Testing and Analysis of Drug Products STERIS Laboratories offers qualitycontrolled services for drug product testing while providing accurate analyses for timesensitive drug products that are to be released and distributed to the commercial marketplace We are experienced in drug product analysis of all types of pharmaceutical products, including solid dosage forms (tablets, capsules, etc), liquids and semisolid products.
ADOH has a network of subcontractors that can take care of the analytical and microbial testing of the samples. Testing categories Batch testing of biological medicines Batches of biological medicines (eg vaccines, insulin and blood products) are tested for quality These medicines are commonly tested before a batch is released;. Independent batch release testing of COVID19 (coronavirus) vaccines As is the case for current, licensed vaccines, the quality of each batch of any potential COVID19 vaccine will be evaluated by an independent laboratory The independent laboratory will also carry out a thorough review of the manufacturer batch documentation that describes.
In other words, samples taken at the manufacturing site, and further samples taken at the site of importation In both cases, the. Batch Release Testing Any facility that will conduct European batch release testing must be identified in the Marketing Authorization Application (MAA) for the product FDAS is a skilled contract testing laboratory that can handle all of your necessary quality control testing to release a medicinal batch for sale. Batch release testing and EU retests Conducting batch release analysis on behalf of a client is synonymous with partnership, communication, trust and control – but also with the many opportunities that become available for the design of the project.
Batch Release and Stability Lot Testing We provide the resources and flexibility to develop and improve analytical methods tailored to your specific needs Our laboratory team has extensive experience in designing and executing protocols for method development and validation, method verification and method transfer — covering procedures for active pharmaceutical ingredients, drug formulations, cosmetics, personal care products and raw materials. This states ”When a fully validated terminal sterilisation method by steam, dry heat or ionising radiation is used, parametric release, that is the release of a batch of sterilised items based on process data rather than on the basis of submitting a sample of the items to sterility testing, may be carried out, subject to the approval of the competent authority”. BATCH RELEASE MRA NO MRA RETESTING REQUIRED FOR BATCH RELEASE BATCH IS RELEASED FOR SALE OR SUPPLY/EXPORT PROBLEMS ADDRESSED LIFE SCIENCE I TECHNICAL BULLETIN 3 that have an MRA with the EU6 If a medicinal product batch is imported in separate parts, either conditions of.
Hi, A batch is 10,000 We actually run every week on this product (its tabletting) The issue i have is that to test each tablet takes 4 minutes and is a destructive test on a very expensive product. The batch release method is a rapid and costeffective approach to sterilizing single or small batches of product needed for clinical or animal trials with minimal sample requirements The overkill concept using Ethylene Oxide (EO) sterilization is used to demonstrate a sterility assurance level to safely release product. In this post you’ll find the first in a series of ‘frequently asked questions’ related to the updated EU GMP Annex 16 on QP Certification and Batch Release I have been a GMDP inspector since October 14, and part of my role is to perform inspections of manufacturers, importers and batch certification sites.
Guidance on the certification by a Qualified Person (QP) and on batch release within the European Union (EU) of medicinal products GMP SEARCH ENGINE Search in GMP Database Training & Conference OnDemand Training Guidelines News & Press Conference folders. Lot release of vaccines by, as a minimum, review of a summary protocol and access to a laboratory are two of the essential functions of a national regulatory authority for assuring the quality of vaccines used in the immunization programme as defined by WHO Lot release is the process of evaluating. Batch release testing helps to ensure the safety and effectiveness of licensed biological medicines, such as vaccines and products derived from human blood products or plasma Every batch of biological medicine produced by a manufacturer must undergo rigorous and independent testing before it can be released on to the market for human use.
This refers to parallel QC testing of samples from the same batch;. Batch release testing programs may include differing combinations of microbial testing, biochemical analysis, purity, safety, and potency testing, as required by the regulatory authority for market entry Technology Transfer. Each year the IRS accepts many tax returns before their official start date These returns are processed early and in most cases they receive their refund a week before individuals not in the test batch This is for testing their Where’s My Refund website as well as testing all of the various processing systems that are in affect.
Batch release QC and QP Do you require chemical or microbiological Quality Control (QC) testing to complete GMP product release?. Batch Release Testing of Medical Devices Smithers offers testing to support batch release of medical devices based on functional requirements of the device or its packaging Our experts have a wealth of experience in physical device testing, materials testing and chemical analysis and can provide you with a comprehensive overview of your product’s performance. (a) For each batch of drug product, there shall be appropriate laboratory determination of satisfactory conformance to final specifications for the drug product, including the identity and strength of each active ingredient, prior to releaseWhere sterility and/or pyrogen testing are conducted on specific batches of shortlived radiopharmaceuticals, such batches may be released prior to.
The batch release method is a rapid and costeffective approach to sterilizing single or small batches of product needed for clinical or animal trials with minimal sample requirements The overkill concept using Ethylene Oxide (EO) sterilization is used to demonstrate a sterility assurance level to safely release product. When batch retesting is required, a skilled contract laboratory can handle all of the quality control testing and valida tion necessary to release a medicinal batch for sale in the European Union. ADOH has a network of subcontractors that can take care of the analytical and microbial testing of the samples.
ATMP News 08/10/19 ATMP from Third Countries New Q&A Paper on Batch Release published Back to overview In a threepage document from July, the European Medicines Agency (EMA) commented on the possibilities for ATMP imports from a third country into the EU to refrain from a new batch testThe present Questions and Answers catalogue first clarifies the fact that the responsible QP is. Lot release is a mechanism that provides FDA with a realtime system to continuously monitor product quality, through review and testing, of many of the biological products that it regulates. Biopharmaceutical Release Testing To support your commercial product and clinical trial material release testing needs, Eurofins BioPharma Product Testing offers the capacity and breadth of capabilities to test your formulated bulk, final product or inprocess materials in a timely manner We test materials against specifications for identity, purity, potency, impurities, physical properties and safety under strict cGMP compliance, and we customize individual programs to streamline lab.
Batch Release Testing Within the European Union (EU), Good Manufacturing Practice (GMP) for Medicinal Products requires batch release against the approved product specification, for medicinal products holding a marketing authorisation. The demand for Batch Testing and Release (T&R) services has experienced a 5fold increase during the last couple of years, possibly due to a lack of trust in the UK Government mitigating measures postBrexit. Before any batch can be released for deployment, the NIBSC will issue a certificate confirming that the independent testing has been performed and that the batch is compliant with the relevant.
Following batch release (batch monitoring) periodically (usually as part of a survey of product groups). The EU batch release and import of biological IMPs (cell therapy and “others”) the EU batch release and import of veterinary sterile and nonsterile medicinal products;. Any facility that will conduct European batch release testing must be identified in the Marketing Authorization Application (MAA) for the product FDAS is a skilled contract testing laboratory that can handle all of your necessary quality control testing to release a medicinal batch for sale Within the European Union (EU), there is a regulatory requirement that each batch of a marketed product is tested by an EU accredited cGMP testing laboratory against the approved product release.
Batch Release Testing and EU Retests Conducting batch release analysis on behalf of a client is synonymous with partnership, communication, trust and control – but also with the many opportunities that become available for the design of the project QPcertified batch release analysis. Quality Control testing for drug release of pharmaceuticals and biopharmaceuticals in the EU and across the globe Good Manufacturing Practice for Medicinal Products require batch release within the European Community (EC) or European Economic Area (EEA) of medicinal products holding a marketing authorisation or made for export. Legal framework As part of the regulation of biological medicinal products, Article 114 of Directive 01//EC relating to medicinal products for human use, as amended by Directive 04/27/EC, of the European Parliament and of the Council provides that a member state laboratory may, but is not required to, test a batch of an immunological medicinal product or a medicinal product derived from.
A) Each year the IRS accepts many tax returns before their official start date These returns are processed early and in most cases they receive their refund a week before individuals not in the test batch. Batch Release Pharmacopoeia Tests (USP, BP, and IP) Pharmacopoeia standards are publicly available and legally enforceable standards of quality for medicinal products and their constituents, used by individuals and organizations involved in pharmaceutical research, development, manufacture and testing These standards are used by regulatory agencies and manufacturers to ensure that these products are of the appropriate identity, as well as strength, quality, purity, and consistency. BATCH RELEASE TESTING BHP provides testing and chemical analysis services for the pharmaceutical industry We test and analyze raw materials and finished products for physical, mechanical and chemical parameters to USP, EP and BP specifications We provide solvent testing to ASTM, IMPCA and customer defined specifications using such techniques as WET CHEMISTRY, GC, GCMS, GCMSMS, LCMS, FTIR, Ion Chromatography and ICPMS analysis.
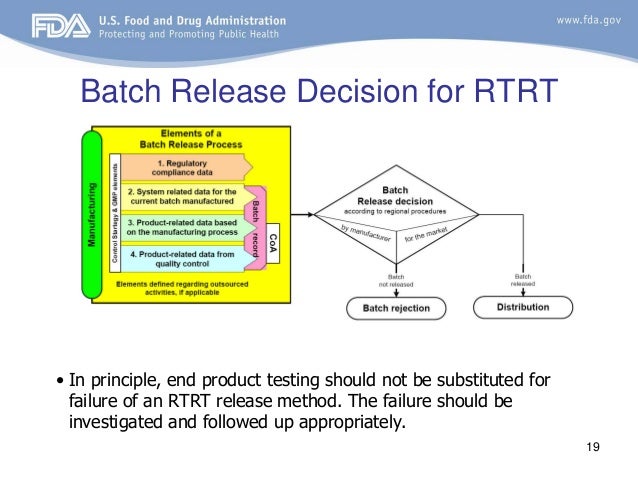
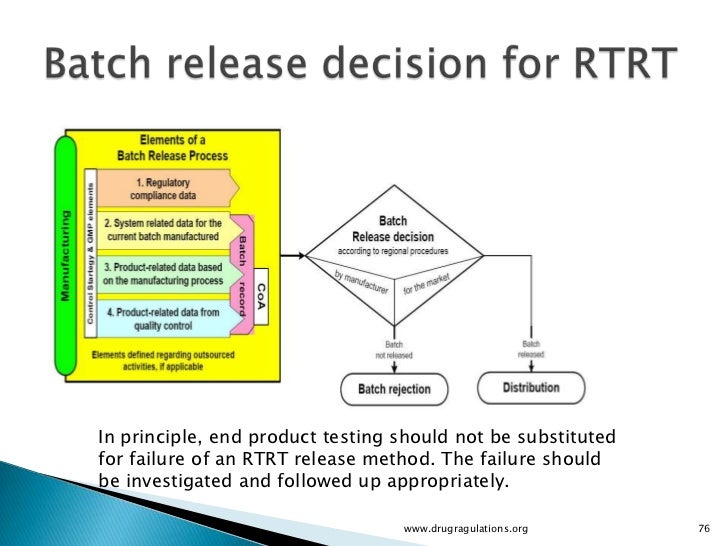
When RTRT has been approved this should be routinely used for batch release In the event that the test results of RTRT fail or are trending toward failure, RTRT may not be substituted by endproduct testing Any failure should be investigated and trending should be followed up appropriately Batch. Our manufacturing department is complemented by a wellequipped chemical and microbiological Quality Control (QC) testing facility. Batch Release Testing and Analysis of Drug Products STERIS Laboratories offers qualitycontrolled services for drug product testing while providing accurate analyses for timesensitive drug products that are to be released and distributed to the commercial marketplace We are experienced in drug product analysis of all types of pharmaceutical products, including solid dosage forms (tablets, capsules, etc), liquids and semisolid products.
Monday 22 June The NC3Rs today announces a new project to review requirements for the use of animals in World Health Organization (WHO) guidelines for the quality control and batch release testing of biological therapeutics and vaccines Cofunded by the Bill & Melinda Gates Foundation, the aim is to help enable manufacturers, regulators, and national control laboratories to apply the latest nonanimal testing approaches and strategies to support faster access to vaccines globally. Batch Release Testing Within the European Union (EU), Good Manufacturing Practice (GMP) for Medicinal Products requires batch release against the approved product specification, for medicinal products holding a marketing authorisation Our QC batch release testing laboratories utilise a wide range of analytical technologies to provide. Batch Release Services According to EU regulations, medicinal products imported into the European Economic Area (EEA) from third countries have to be certified by a Qualified Person (QP) to be released into the market.
Good Manufacturing Practice (GMP) batch release testing is a necessary requirement to ensure high quality pharmaceuticals and biopharmaceuticals prior to release for sale, supply or export Whilst you focus on your core business activities, you will need a contract analytical services partner with a strong history of delivering regulatory compliant API, IMP or finished products batch release testing to a consistently high standard with responsive turnaround times. We conduct preregistration assessment and testing to develop and validate test methods in our laboratory, to ensure prompt and efficient batch release testing We may consult you regarding testing procedures during this time Risk Group 2 Full batch release Your product will fall into full batch release (Risk Group 2) if it is. We found 41 results for Batch Release Testing For more results try searching for Batch Release Testing across all experimental services experiment Batch Release Testing COVID19 Research COVID19 specific reagent(s), models and discounts sector Commercial Certified/Qualified.
The USP content uniformity (CU) test methodology for batch release includes testing of individual doses of finished pharmaceuticals to ensure that the product meets quality specifications 8 Testing starts during manufacturing, when at least 30 dosage units are sampled, usually as a random composite from the batch, and includes up to two stages of analytical testing to determine conformance to USP. Batch Release Testing Batch Release Testing A hallmark of highquality pharmaceutical and biopharmaceutical products is regular quality Our Batch Release Testing Capabilities CPT Labs has over 40 years of experience in consumer product testing and Batch Release Testing Outline Raw materials. BATCH RELEASE MRA NO MRA RETESTING REQUIRED FOR BATCH RELEASE BATCH IS RELEASED FOR SALE OR SUPPLY/EXPORT PROBLEMS ADDRESSED LIFE SCIENCE I TECHNICAL BULLETIN 3 that have an MRA with the EU6 If a medicinal product batch is imported in separate parts, either conditions of.
Legal framework As part of the regulation of biological medicinal products, Article 114 of Directive 01//EC relating to medicinal products for human use, as amended by Directive 04/27/EC, of the European Parliament and of the Council provides that a member state laboratory may, but is not required to, test a batch of an immunological medicinal product or a medicinal product derived from. A new project to reduce animal use in the batch release and quality control testing of biologicals Monday 22 June The NC3Rs today announces a new project to review requirements for the use of animals in World Health Organization (WHO) guidelines for the quality control and batch release testing of biological therapeutics and vaccines. Samples of the batch to be released are sent, along with production and control protocols, to an OMCL within the EU/EEA If the results are satisfactory, the Competent Authority (CA) issues an "Official Control Authority Batch Release Certificate" to the MAH.
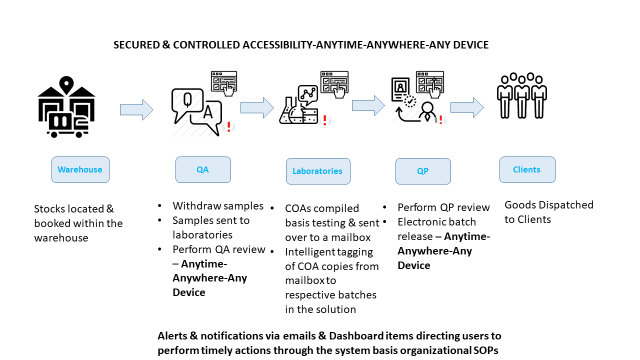
The EU batch release and import of veterinary sterile and nonsterile medicinal products ADOH has a network of subcontractors that can take care of the analytical and microbial testing of the samples ADOH service also includes GMP auditing of API manufacturers and if necessary GMP support for your finished product manufacturing facilities. What is the Test Batch?. QC Testing Contract Analytical Testing We offer QC testing testing & batch release for finished products on Specials Pharmaceuticals Cosmetics Veterinary Herbals Nutriceuticals 10day turnaround times Our team has a lot of experience in the analysis of finished products, and we understand the pressures faced with getting stock.
Batch Release & Stability Testing Dedicated Support Our scientists utilize the latest instrumentation and methodologies to provide customers with the highest quality of analytical analysis of MDI’s, DPI’s, nebulizers, and nasal drug products. There are two testing processes once a batch of the vaccine is produced – by the manufacturer itself, and by the independent National Institute for Biological Standards and Control (NIBSC), part of the MHRA It is only when both of these processes are complete that the batch can be released for use. (a) For each batch of drug product, there shall be appropriate laboratory determination of satisfactory conformance to final specifications for the drug product, including the identity and strength of each active ingredient, prior to release Where sterility and/or pyrogen testing are conducted on specific batches of shortlived radiopharmaceuticals, such batches may be released prior to completion of sterility and/or pyrogen testing, provided such testing is completed as soon as possible.

Batch Release Testing And Eu Re Tests Dsi Pharm

Sam Lowe So Uk Asing For Mutual Recognition Of Good Manufacturing Practice In Medical Products And Mutual Recognition Of Batch Testing Certificates But Not An Exemption From Needing An Eu Based

Nadlezhashaya Proizvodstvennaya Praktika
Batch Release Testing のギャラリー
Rtrt Regulatory Perspectiv On Real Time Release Testing 27 Oct 11
2

Annex 2 Good Trade And Distribution Practices For Pharmaceutical Starting Materials Pdf Free Download

Aseptic Fill And Finish Eurofins Biopharma Product Testing Nl

A Summary Table Demonstrating Batch Release Testing Results Of Opregen Download Table

Batch Release Testing Als Testing
Real Time Release Testing

Batch Release Testing Als Life Sciences Europe
Leverage Technology For Timely Batch Release Ensuring Compliancesupply Chain And Sd

Guideline On Real Time Release Testing Ipq
Thread By Jamescrisp6 Eu Accused Of Risking Lives In Standoff Over Coronavirus Medicines Produced In

The Economic Efficiency Of Small Batch Software Applause
Paul Ehrlich Institut Press Releases Paul Ehrlich Institut Releases First Batches Of Biontech Pfizer S Covid 19 Vaccine Comirnaty For German And European Markets

Biologics And Biosimilar Gmp Release Testing

Analytical Laboratory Services Market And Government Initiatives To Strengthen Analytical Testing Capabilities Teletype
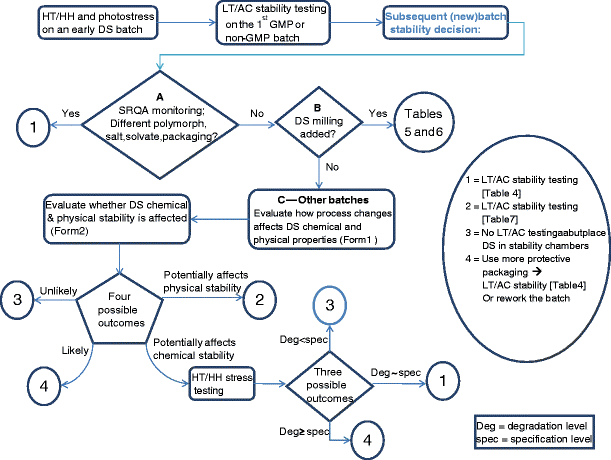
Best Practices For Drug Substance Stress And Stability Studies During Early Stage Development Part Iii How To Make Science And Risk Based Stability Testing Decisions For Drug Substance Batches Produced After Manufacturing Process Changes

Import Testing Ifpma
Http Www Sciencedirect Com Science Article Pii Sx Pdf Md5 93e711a94a70f077cb034e6bb Pid 1 S2 0 Sx Main Pdf
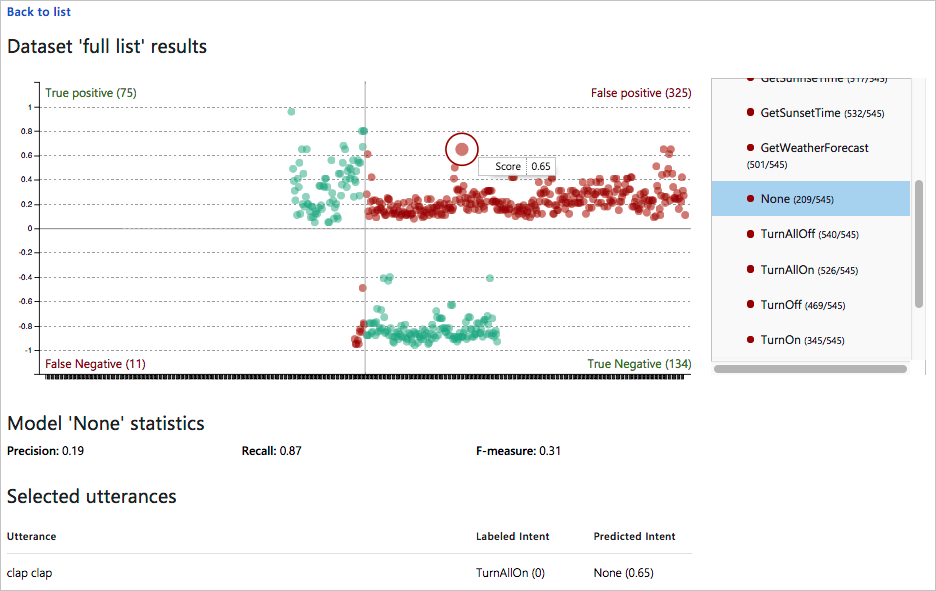
Een Batch Test Uitvoeren Luis Azure Cognitive Services Microsoft Docs
2

Batch Release Qc And Qp Eurofins Biopharma Product Testing Nl
Achieving Compliant Batch Release Sterile Parenteral Quality Control Beckman Coulter

Running Qs Regression Test In Batch Qlikview Cookbook

Ph21 Software Charles Ischi Ag

Batch Testing And Release Life After Brexit Asphalion
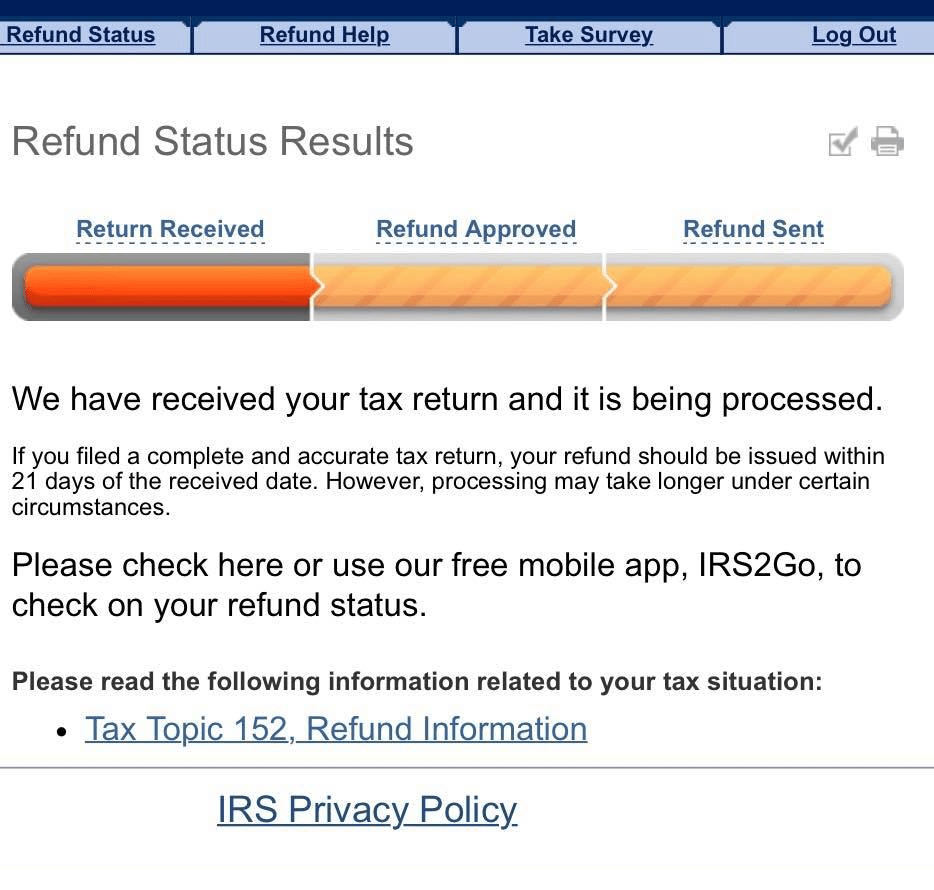
Did Your Tax Return Get Selected To Take Part In The Irs Test Batch This Year Refundtalk Com

Independent Batch Release Testing Of Covid 19 Coronavirus Vaccines By The Nibsc Gov Uk

Batch Release Testing Tepnel Pharma Services

Sampling And Testing In Exhibit And Process Validation Batches Sampling Statistics Science Mathematics

Tentamus Group Gmbh With Brexit Coming Up Many Pharmaceutical Companies Will Need A Partner For Getting Their Products To Market Who Can You Rely On To Support You Through This Click

5 25 How To Do Automated Batch Testing
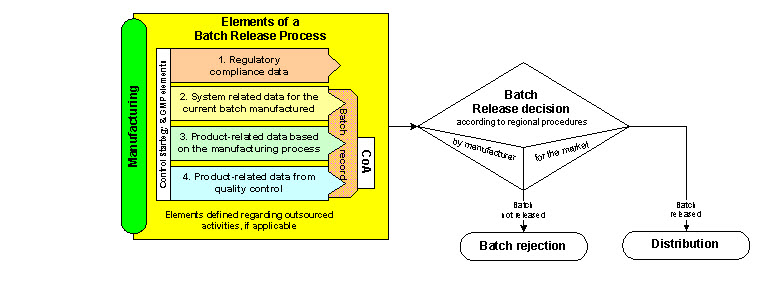
Q8 Q9 Q10 Questions And Answers Appendix Q As From Training Sessions Q8 Q9 Q10 Points To Consider Fda

14 Businesses Doing A Great Job At Batch Release Testing Membranefiltrationsterilitytestinglryb700 Over Blog Com
2

Laboserve The One Stop Shop Service Provider In Healthcare Business Laboserve Eu

Stockport Quality Control North West Batch Release Testing

Eu Offers Flexibility On Uk Batch Release In No Deal Brexit Scenario Pink Sheet

Real Time Release Testing
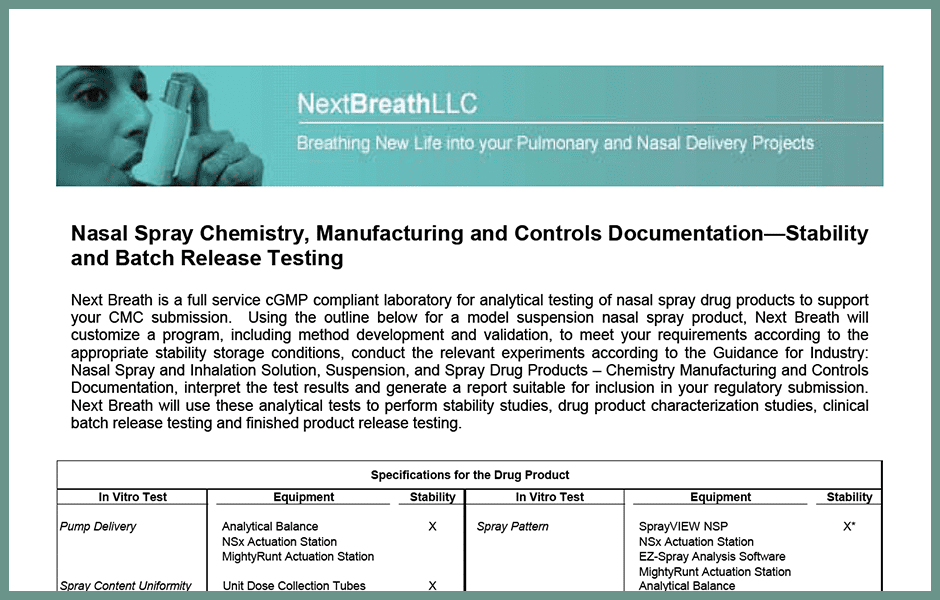
Nasal Spray Stability And Batch Release Testing Next Breath

Figure 1 3 From Tools For Real Time Release Testing Rtrt In Batch And Continuous Tablet Manufacturing Semantic Scholar
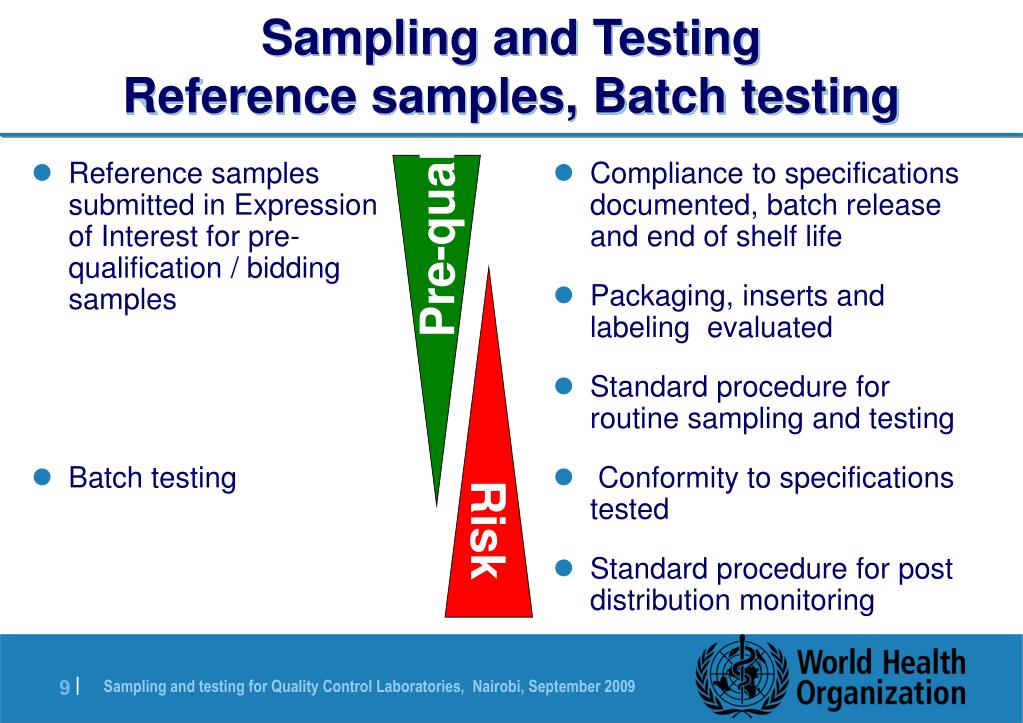
Ppt Quality Control Testing In Procurement Powerpoint Presentation Free Download Id 8172

European Commission Brussels Date

Latest Batch Of Port Employees All Test Negative For Covid 19 Five Port Employees Remain In Isolation Port Authority Of Guam

Real Time Release Testing

Qc Batch Release Testing
Batch Release Test Lab Testing Services ल ब र टर ट स ट ग सर व स In Rasoolpura Chennai Vanta Bioscience Limited Id

Drivers And Barriers In The Consistency Approach For Vaccine Batch Release Testing Report Of An International Workshop

Batch Testing And Release Of Medicinal Products Imported From A

Download Final Approach Batch Meaning Hockeyfasr
Batch Testing Kore Ai Documentation V7 3
Example Results From Batch Vfa Uptake Op Release Testing Showing A Download Scientific Diagram

Ongoing Stability Testing For Listed And Complementary Medicines Therapeutic Goods Administration Tga
Biologics Biopharmaceutical Testing Bioanalysis And Immunogenicity Biologics Characterization

Rsdc Nsdc Lab Chemist Batch Release Testing Mock Test
Modeling Performance Tests

Gmp Logfile Lead Article Gmp Verlag Logfile No 39 18 Batch Release Process Steps

What Happens To Batch Testing And Batch Release After Brexit Kymos Pharma Services
Extranet Who Int Pqweb Key Resources Documents Vaccines Testing

Incremental Gmps For Imps And Qp Batch Certification Ppt Download

New Release Analytical Laboratory Services Market Worth Usd 333 8 Million By 21 By Healthcare Issuu

Benefits Of Small Batch Testing
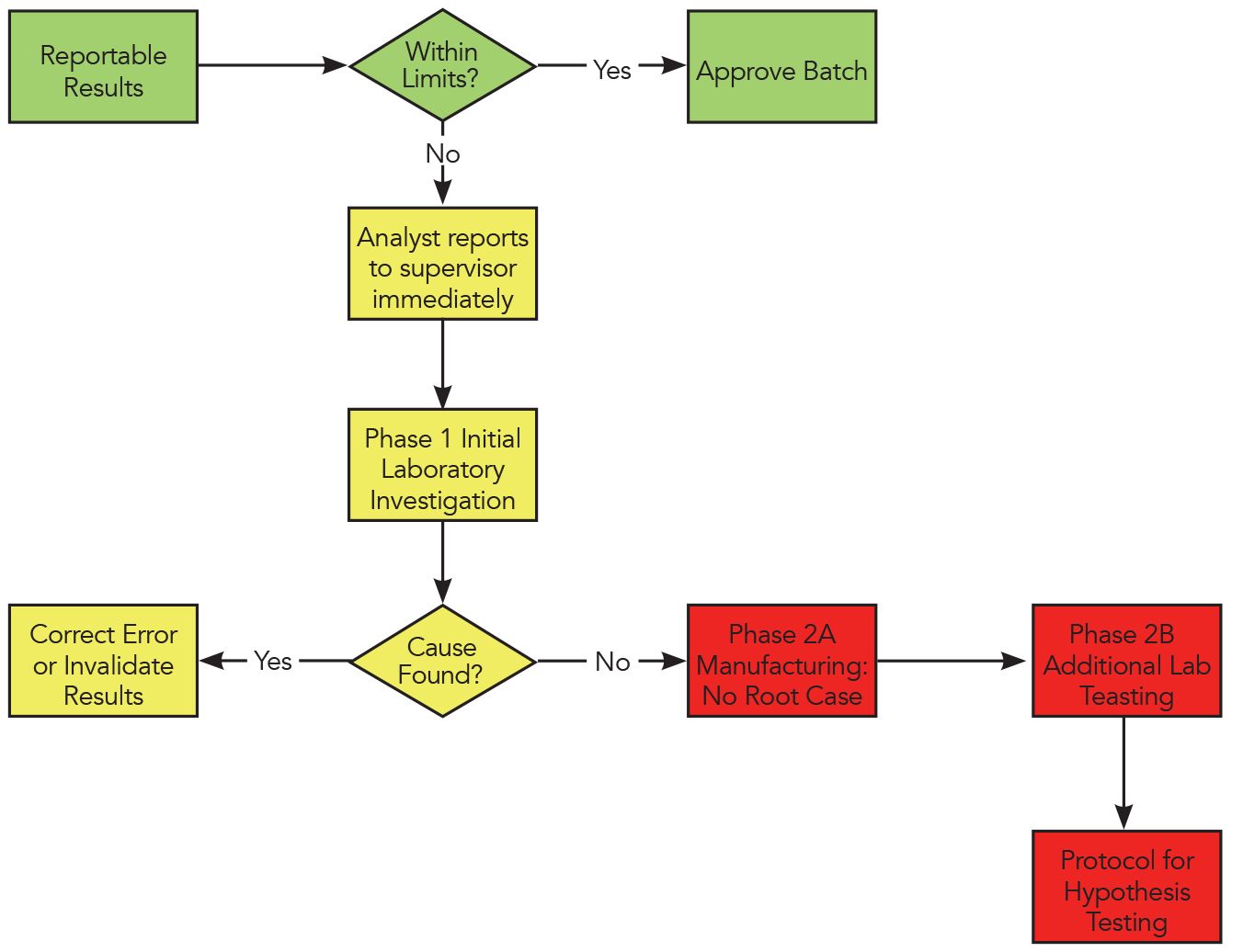
Are You Invalidating Out Of Specification Oos Results Into Compliance Chromatography Online
Www Sgsgroup Us Com Media Global Documents Technical Documents Sgs Lab Batch Retest En 11 Pdf
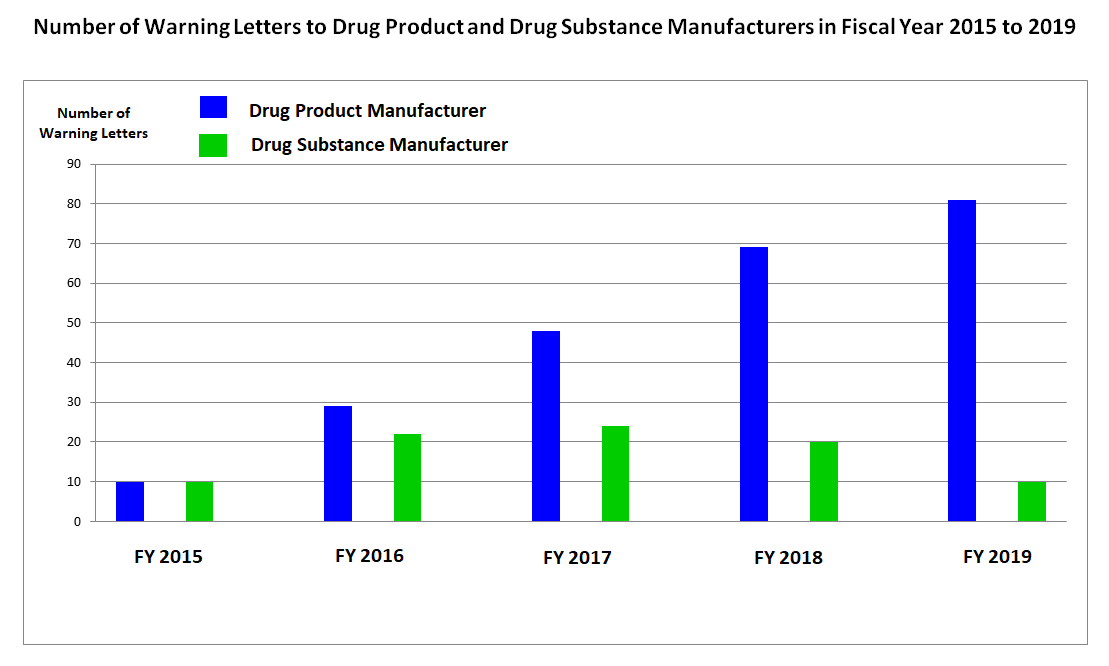
Batch Release Without Determination Of Identity And Strength And Other Gmp Violations A Look At Fda S Warning Letters Over The Last Months Eca Academy
2
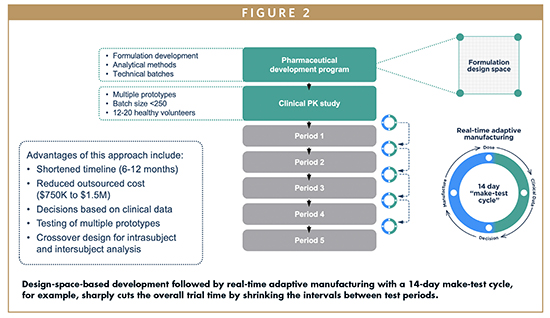
Modified Release Alternative Strategies For Development Of Modified Release Dosage Forms

Bpr Review And Batch Release
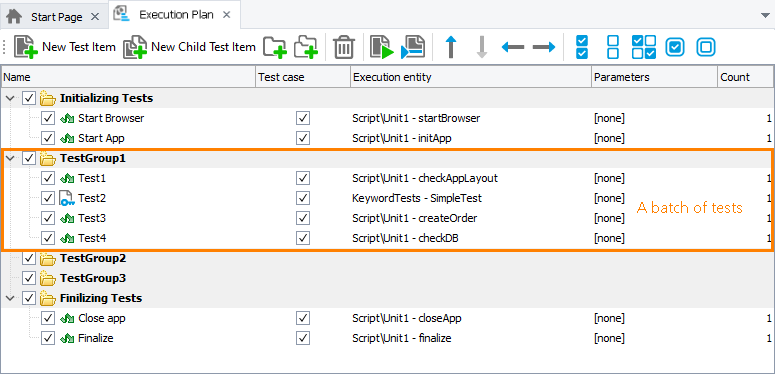
Creating Batch Test Runs Testcomplete Documentation

Benefits Of Small Batch Testing

Iso Ts 1 En Assessment Of Conformity Of Plastics Piping Systems For The Rehabilitation Of Existing Pipelines Part 1 Polyethylene Pe Material

Batch Testing And Release Life After Brexit Asphalion

Gmp Pharmaceutical Batch Release Testing
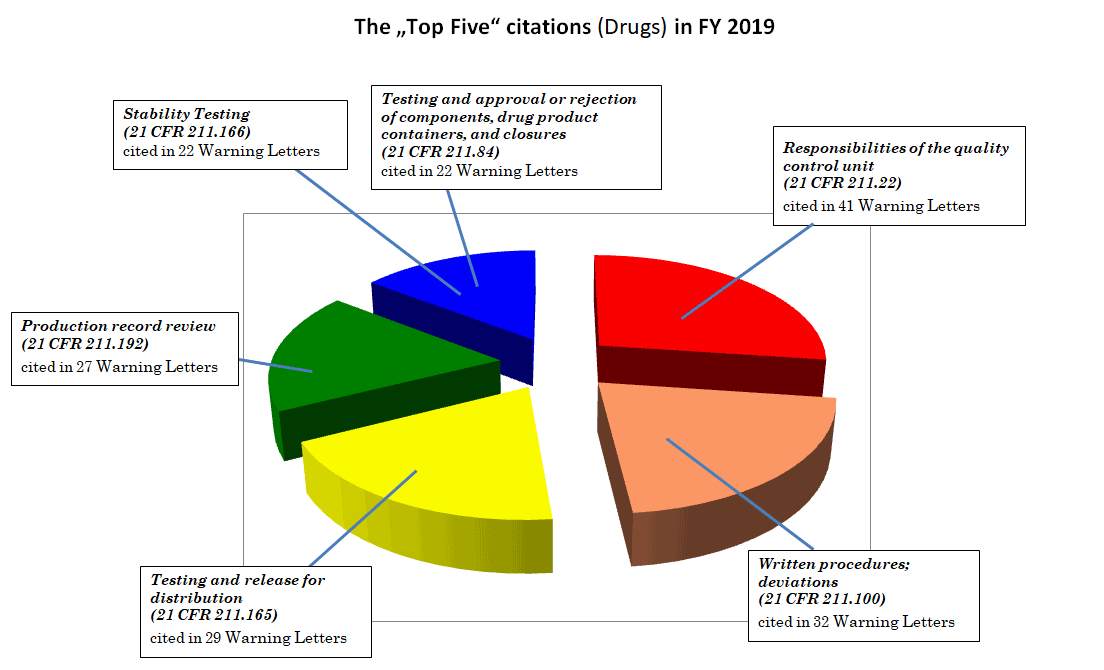
Batch Release Without Determination Of Identity And Strength And Other Gmp Violations A Look At Fda S Warning Letters Over The Last Months Eca Academy

Pdf Lot Release Of Vaccines By Regulatory Authorities And Harmonization Of Testing Requirements
Science Vla Gov Uk Tse Lab Net Documents Tse Eurlpdb01 Pdf
Assets Publishing Service Gov Uk Government Uploads System Uploads Attachment Data File 4316 5152 V8 Animal Usage For Qc Batch Release Of Ivmps 07 12 Pdf

Figure 1 1 From Tools For Real Time Release Testing Rtrt In Batch And Continuous Tablet Manufacturing Semantic Scholar
Www Ema Europa Eu En Documents Other Questions Answers Exemption Batch Controls Carried Out Atmps Imported European Union Third Country En Pdf

Batch Testing Batch Release Services

Stc Stc Tested Mark Certification For First Batch Of 16 Face Mask Manufacturers To Gain Confidence Of Consumers 上海标检产品检测有限公司

Small Batch Testing A Small Change To Your Process A Big Change For Results Seer Interactive

Fillable Online Qwikchecktm Test Strips Website Batch Release Form Fax Email Print Pdffiller
Diving Into Batch To Batch Variability Of Topical Products A Regulatory Bottleneck Pharmaceutical Research X Mol

Batch Testing Batch Release Kymos Pharma Services S L Cphi Online

A Summary Table Demonstrating Batch Release Testing Results Of Opregen Download Table

Omnicell Are You At Ashp17 Don T Miss Our Educational Iv Symposium With Thomas C Kupiec Phd President And Ceo Of Arl Bio Pharma On Extending Beyond Use Dating Batch Release

Real Time Release Testing
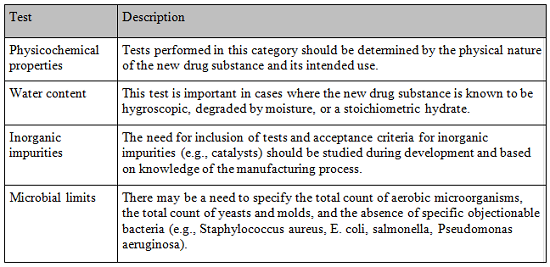
Considerations For Biologic Drug Substance And Drug Product Testing
What S In A Name Chromatography Online

Case Study Ppt Download

Batch Size Reduction And Software Testing Duncan Nisbet Software Delivery Consultant
Cjthissjdg0m
Vichsec Org En Component Attachments Attachments 1678 Html Task Download

Tga Laboratories Testing Report Seasonal Influenza Vaccines Batch Release Therapeutic Goods Administration Tga

Smart Fifo In A Qc Lab Planning With Binocs Scheduler Binocs

Testing Of Biological Medicines

Table 2 From Best Practices For Drug Substance Stress And Stability Studies During Early Stage Development Part Iii How To Make Science And Risk Based Stability Testing Decisions For Drug Substance Batches Produced After




